The Barton decarboxylation is a radical reaction in which a carboxylic acid is first converted to a thiohydroxamate ester (commonly referred to as a Barton ester). The product is then heated in the presence of a radical initiator and a suitable hydrogen donor to complete the reductive decarboxylation of the initial carboxylic acid. Using this reaction it is possible to remove a carboxylic acid moiety from an alkyl group and replace it with other functional groups.
Tuesday, December 27, 2016
Sunday, June 5, 2016
C2c2—the first naturally-occurring CRISPR system that targets RNA, rather than DNA
Researchers unlock new CRISPR system for targeting RNA | Broad Institute of MIT and Harvard
A team led by Feng Zhang of the Broad and MIT and Eugene Koonin of the NIH has revealed that C2c2 helps protect bacteria against viral infection by targeting RNA. Photo composite by Lauren Solomon, Broad communications. Images courtesy of Broad communications and NIH.
In a study published today in Science, Feng Zhang and colleagues at the Broad Institute and the McGovern Institute for Brain Research at MIT, along with co-authors Eugene Koonin and his colleagues at the NIH, and Konstantin Severinov of Rutgers University-New Brunswick and Skoltech, report the identification and functional characterization of C2c2, an RNA-guided enzyme capable of targeting and degrading RNA.
Abudayyeh, Omar O., et al. "C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector." bioRxiv (2016): 054742.
Clustered regularly interspaced short palindromic repeats (CRISPR, pronounced crisper) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacteriophage virus or plasmid. The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign genetic elements such as those present within plasmids and phages, and provides a form of acquired immunity. CRISPR spacers recognize and cut these exogenous genetic elements in a manner analogous to RNA interference in eukaryotic organisms. CRISPRs are found in approximately 40% of sequenced bacterial genomes and 90% of sequenced archaea.
Friday, June 3, 2016
Chemicalize.org - "google search" with structures
 |
| The Methotraxate's "chemicalized" page on Wikipedia. (Photo credit: Wikipedia) |
Interesting idea, but they have to make this tool more accessible to the people (for example - to make an extension to browser).
Chemicalize.org is a free chemical structure miner and web search engine developed and owned by ChemAxon. The main purpose of chemicalize.org is to identify chemical names (SMILES, traditional and IUPAC names) on websites and convert them to chemical structures. chemicalize.org provides other services such as structure based predictions, chemical search, and a “chemicalized” web search.
Further reading:
[PDF] Extracting and connecting chemical structures from text sources usingchemicalize. org.
C Southan, A Stracz - J. Cheminformatics, 2013
Cited by 10
Thursday, June 2, 2016
UniProte database
 |
| Protein image from OPM database (Photo credit: Wikipedia) |

Further reading:
The universal protein resource (UniProt)
AM Bairoch, R Apweiler, CH Wu, WC Barker… - Nucleic acids research, 2005
Cited by 1569
The gene ontology annotation (goa) database: sharing knowledge in uniprot with gene ontology
E Camon, M Magrane, D Barrell, V Lee, E Dimmer… - Nucleic acids research, 2004
Cited by 785
UniProt: the universal protein knowledgebase
R Apweiler, A Bairoch, CH Wu, WC Barker… - Nucleic acids research, 2004
Cited by 1488
Fluorophore molecules - wiki
Non-protein organic fluorophores belong to following major chemical families:
Xanthene derivatives: fluorescein, rhodamine, Oregon green, eosin, and Texas red
 Xanthene (9H-xanthene, 10H-9-oxaanthracene) is a yellow organicheterocyclic compound. Its chemical formula is C13H10O. It is soluble in diethyl ether. Its melting point is 101-102 °C and its boiling point is 310-312 °C. Xanthene is used as a fungicide and it is also a useful intermediate in organic synthesis.
Xanthene (9H-xanthene, 10H-9-oxaanthracene) is a yellow organicheterocyclic compound. Its chemical formula is C13H10O. It is soluble in diethyl ether. Its melting point is 101-102 °C and its boiling point is 310-312 °C. Xanthene is used as a fungicide and it is also a useful intermediate in organic synthesis.

Cyanine is a non-systematic name of a synthetic dye family belonging topolymethine group. The word cyanin is from the English word “cyan", which conventionally means a shade of blue-green (close to "aqua") and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue".
 Squaraine dyes are a class of organic dyes showing intense fluorescence, typically in the red and near infrared region (absorption maxima are found between 630 and 670 nm and their emission maxima are between 650–700 nm). They are characterized by their unique aromatic four membered ring system derived from squaric acid. Most squaraines areencumbered by nucleophilic attack of the central four membered ring, which is highly electron deficient. This encumbrance can be attenuated by the formation of arotaxane around the dye to protect it from nucleophiles. They are currently used as sensors for ions and have recently, with the advent of protected squanaine derivatives, been exploited in biomedical imaging.
Squaraine dyes are a class of organic dyes showing intense fluorescence, typically in the red and near infrared region (absorption maxima are found between 630 and 670 nm and their emission maxima are between 650–700 nm). They are characterized by their unique aromatic four membered ring system derived from squaric acid. Most squaraines areencumbered by nucleophilic attack of the central four membered ring, which is highly electron deficient. This encumbrance can be attenuated by the formation of arotaxane around the dye to protect it from nucleophiles. They are currently used as sensors for ions and have recently, with the advent of protected squanaine derivatives, been exploited in biomedical imaging.
 Naphthalene is an organic compoundwith formula C10H8. It is the simplestpolycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatichydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.
Naphthalene is an organic compoundwith formula C10H8. It is the simplestpolycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatichydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.
Coumarin derivatives

Coumarin (/ˈkuːmərɪn/; 2H-chromen-2-one) is a fragrant organic chemical compound in the benzopyrone chemical class, which is a colorless crystalline substance in its standard state. It is a natural substance found in many plants.

Furazan, or 1,2,5-oxadiazole, is anheterocyclic aromatic organic compoundconsisting of a five-atom ring containing 1 oxygen and 2 nitrogen atoms. The furazan ring system is also found in the steroid furazabol. Furazan and its derivatives are obtained from the oximederivatives of 1,2-diketones.
 Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fusedbenzene rings. It is a component of coal tar. Anthracene is used in the production of the red dyealizarin and other dyes. Anthracene is colorless but exhibits a blue (400-500 nm peak) fluorescence under ultraviolet light.
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fusedbenzene rings. It is a component of coal tar. Anthracene is used in the production of the red dyealizarin and other dyes. Anthracene is colorless but exhibits a blue (400-500 nm peak) fluorescence under ultraviolet light.
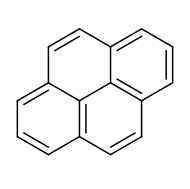
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flataromatic system. The chemical formula is C16H10. This colorless solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds.
Acridine derivatives: proflavin, acridine orange, acridine yellow, etc.
 Acridine is an organic compound and anitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.
Acridine is an organic compound and anitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.
 Auramine O is a diarylmethane dyeused as a fluorescent stain. In its pure form, Auramine O appears as yellow needle crystals. It is very soluble in water and soluble in ethanol.
Auramine O is a diarylmethane dyeused as a fluorescent stain. In its pure form, Auramine O appears as yellow needle crystals. It is very soluble in water and soluble in ethanol.
Tetrapyrrole derivatives: porphin, phthalocyanine, bilirubin
Xanthene derivatives: fluorescein, rhodamine, Oregon green, eosin, and Texas red



Naphthalene derivatives (dansyl and prodan derivatives)







Tetrapyrroles are a class of chemical compounds whose molecules contain four pyrrole rings held together by directcovalent bonds or by one-carbon bridges (=(CH)- or -CH2-units), in either a linear or a cyclic fashion. A pyrrole ring in a molecule is a five-atom ring where four of the ring atoms are carbon and one is nitrogen. In cyclic tetrapyrroles, lone electron pairs on nitrogen atoms facing the center of the macrocycle ring can bond or chelate with a metal ion such as iron,cobalt, or magnesium.
Applications of fluorescence correlation spectroscopy
WW Webb - Quarterly reviews of biophysics, 1976
Cited by 84
[B] Molecular fluorescence: principles and applications
B Valeur, MN Berberan-Santos - 2012
This second edition of the well-established bestseller is completely updated and revised with approximately 30% additional material, including two new chapters on applications,which has seen the most significant developments. The comprehensive overview written ...
Cited by 4817
High-throughput and ultra-high-throughput screening: solution-and cell-based approaches
SA Sundberg - Current opinion in biotechnology, 2000
Cited by 449
Subscribe to:
Posts (Atom)