Zeta potential is a scientific term for electrokinetic potential in colloidaldispersions. In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta (ζ), hence ζ-potential. From a theoretical viewpoint, the zeta potential is the electric potential in the interfacial double layer (DL) at the location of the slipping plane relative to a point in the bulk fluid away from the interface.
Further reading:
Thursday, March 2, 2017
Density Functional Theory (DFT) - wiki
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases.
Further reading:
Further reading:
[C] Density functional theory
M Schlüter, LJ Sham - Physics Today, 1982
Cited by 104
[HTML] Density functional theory
M Orio, DA Pantazis, F Neese - Photosynthesis research, 2009
Cited by 101
Monday, February 13, 2017
Hit to lead (H2L) - wiki
Hit to lead (H2L) also known as lead generation is a stage in early drug discovery where small molecule hits from a high throughput screen (HTS) are evaluated and undergo limited optimization to identify promising lead compounds.[1][2] These lead compounds undergo more extensive optimization in a subsequent step of drug discovery called lead optimization (LO).[3][4] The drug discovery process generally follows the following path that includes a hit to lead stage:
- target validation (TV) → assay development → high-throughput screening → hit to lead (H2L) → lead optimization (LO) → preclinical drug development → clinical drug development
Further reading:
Hit and lead generation: beyond high-throughput screening
KH Bleicher, HJ Böhm, K Müller, AI Alanine - Nature reviews Drug discovery, 2003
Cited by 887
[HTML] Hit discovery and hit-to-lead approaches
GM Keserű, GM Makara - Drug discovery today, 2006
Cited by 189
[HTML] Hit-to-lead studies: the discovery of potent adamantane amide P2X 7 receptor antagonists
A Baxter, J Bent, K Bowers, M Braddock, S Brough… - Bioorganic & medicinal …, 2003
Cited by 109
Abamectin - wiki
Abamectin is a widely used insecticide and anthelmintic.
Abamectin is a mixture of avermectins containing more than 80% avermectin B1a and less than 20% avermectin B1b. These two components, B1a and B1b have very similar biological and toxicological properties. The avermectins are insecticidal and antihelmintic compounds derived from various laboratory broths fermented by the soil bacterium Streptomyces avermitilis. Abamectin is a natural fermentation product of this bacterium.
[HTML] A review of regional and temporal use of avermectins in cattle and horses worldwide
AB Forbes - Veterinary parasitology, 1993
Cited by 48
Evolution of a specific fluorogenic derivatization of ivermectin for bioanalytical applications. A review
DW Fink, JSK Shim - Analyst, 1996
Cited by 13
The Morita-Baylis-Hilman reaction - wiki
The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.
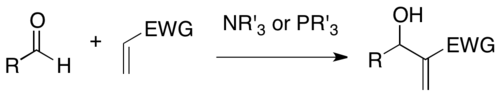
Links:
http://www.organic-chemistry.org/namedreactions/baylis-hillman-reaction.shtm
http://www.name-reaction.com/baylis-hillman-reaction
http://www.name-reaction.com/baylis-hillman-reaction
Further reading:
The Catalyzed α‐Hydroxyalkylation and α‐Aminoalkylation of Activated Olefins (The Morita—Baylis—Hillman Reaction)
E Ciganek - Organic Reactions, 1997
Cited by 776
In a patent application published in 1972, Baylis and Hillman reported the reaction of acetaldehyde with ethyl acrylate and acrylonitrile in the presence of catalytic amounts of 1,4-diazabicyclo[2.2.2]octane to give the α-hydroxyethylated products in good yields. No structure proof was given.
The enantioselective Morita–Baylis–Hillman reaction and its aza counterpart
G Masson, C Housseman, J Zhu - Angewandte Chemie International Edition, 2007
Asymmetric Morita− Baylis− Hillman Reactions Catalyzed by Chiral Brønsted Acids
NT McDougal, SE Schaus - Journal of the American Chemical Society, 2003
Cited by 311

Tuesday, January 24, 2017
MRSA - wiki
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium responsible for several difficult-to-treat infections in humans. MRSA is any strain of Staphylococcus aureus that has developed, through horizontal gene transfer and natural selection, multi- resistance to beta-lactam antibiotics, which include the penicillins (methicillin, dicloxacillin, nafcillin, oxacillin, etc.) and the cephalosporins. MRSA evolved from horizontal gene transfer of the mecA gene to at least five distinct S. aureus lineages
Methicillin-resistant Staphylococcus aureus (MRSA): review
MT File - Southern African Journal of Epidemiology and Infection, 2008
Cited by 11
Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature
BS Cooper, SP Stone, CC Kibbler, BD Cookson… - Bmj, 2004
Cited by 344
Surfactin - wiki
Surfactin is a very powerful surfactant commonly used as an antibiotic. It is a bacterial cyclic lipopeptide, largely prominent for its exceptional surfactant power.[2] Its amphiphilic properties help this substance to survive in both hydrophilic and hydrophobic environments. It is an antibiotic produced by the Gram-positive endospore-forming bacteria Bacillus subtilis.


[HTML] Review of surfactin chemical properties and the potential biomedical applications
G Seydlová, J Svobodová - Open Medicine, 2008
Cited by 117
Surfactin--A Review on Biosynthesis, Fermentation, Purification and Applications.
NS Shaligram, RS Singhal - Food Technology & Biotechnology, 2010
Cited by 78
[HTML] Surfactant-enhanced remediation of contaminated soil: a review
CN Mulligan, RN Yong, BF Gibbs - Engineering Geology, 2001
Cited by 788
Vibrio cholerae - wiki
Vibrio cholerae is a Gram-negative, comma-shaped bacterium. The bacterium's natural habitat is brackish or saltwater. Some strains of V. cholerae cause the disease cholera. V. cholerae is a facultative anaerobe[1] and has a flagellum at one cell pole as well as pili. V. cholerae can undergo respiratory and fermentative metabolism. When ingested, V. cholerae can cause diarrhea and vomiting in a host within several hours to 2–3 days of ingestion. V. cholerae was first isolated as the cause of cholera by Italian anatomist Filippo Pacini in 1854,[2] but his discovery was not widely known until Robert Koch, working independently 30 years later, publicized the knowledge and the means of fighting the disease
Non-O: l Vibrio cholerae bacteremia: case report and review
S Safrin, JG Morris, M Adams, V Pons, R Jacobs… - Review of Infectious …, 1988
Cite
[PDF] The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review
MS Islam, BS Drasar, RB Sack - Journal of diarrhoeal diseases research, 1994
Cite
[HTML] Quorum sensing controls biofilm formation in Vibrio cholerae
BK Hammer, BL Bassler - Molecular microbiology, 2003
PAMAM - wiki
Poly(amidoamine), or PAMAM, is a class of dendrimer which is made of repetitively branched subunits of amide and amine functionality. PAMAM dendrimers sometimes referred to by the trade name Starburst, have been extensively studied since their synthesis in 1985, and represent the most well-characterized dendrimer family as well as the first to be commercialized.
 | |
|
[HTML] Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications
R Esfand, DA Tomalia - Drug discovery today, 2001
Cited by 1307
[HTML] The influence of surface modification on the cytotoxicity of PAMAM dendrimers
R Jevprasesphant, J Penny, R Jalal, D Attwood… - International journal of …, 2003
Cited by 563
[PDF] Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers
JC Roberts, MK Bhalgat, RT Zera - Journal of biomedical materials research, 1996
Cited by 557
Subscribe to:
Posts (Atom)

