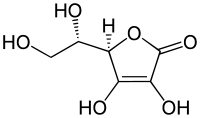
 |
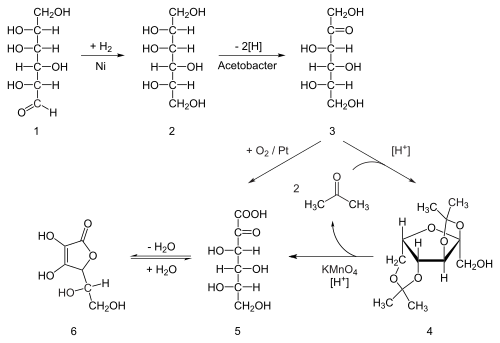
Ascorbic acid is prepared in industry from glucose in a method based on the historical Reichstein process. In the first of a five-step process, glucose is catalytically hydrogenated to sorbitol, which is then oxidized by themicroorganism Acetobacter suboxydans to sorbose. Only one of the six hydroxy groups is oxidized by this enzymatic reaction. From this point, two routes are available. Treatment of the product with acetone in the presence of an acid catalyst converts four of the remaining hydroxyl groups to acetals. The unprotected hydroxyl group is oxidized to the carboxylic acid by reaction with the catalytic oxidant TEMPO (regenerated by sodium hypochlorite —bleaching solution). Historically, industrial preparation via the Reichstein process used potassium permanganate as the bleaching solution. Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization. This step yields ascorbic acid. Each of the five steps has a yield larger than 90%.
A more biotechnological process, first developed in China in the 1960s, but further developed in the 1990s, bypasses the use of acetone-protecting groups. A second genetically modified microbe species, such as mutant Erwinia, among others, oxidises sorbose into 2-ketogluconic acid (2-KGA), which can then undergo ring-closing lactonization via dehydration. This method is used in the predominant process used by the ascorbic acid industry in China, which supplies 80% of world's ascorbic acid.[30] American and Chinese researchers are competing to engineer a mutant that can carry out a one-pot fermentation directly from glucose to 2-KGA, bypassing both the need for a second fermentation and the need to reduce glucose to sorbitol.
The Reichstein process in chemistry is a combined chemical and microbial method for the production of ascorbic acid from D-glucose that takes place in several steps. This process was devised by Nobel Prize winner Tadeus Reichstein and his colleagues in 1933 while working in the laboratory of the ETH in Zürich.

Tadeusz Reichstein (20 July 1897 – 1 August 1996) was a Polish bornchemist, naturalized Swiss and the Nobel Prize in Physiology or Medicinelaureate (1950).


Further reading:
I was diagnosed as HEPATITIS B carrier in 2013 with fibrosis of the
ReplyDeleteliver already present. I started on antiviral medications which
reduced the viral load initially. After a couple of years the virus
became resistant. I started on HEPATITIS B Herbal treatment from
ULTIMATE LIFE CLINIC (www.ultimatelifeclinic.com) in March, 2020. Their
treatment totally reversed the virus. I did another blood test after
the 6 months long treatment and tested negative to the virus. Amazing
treatment! This treatment is a breakthrough for all HBV carriers.