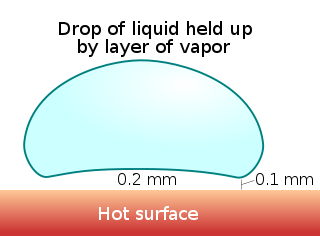
The Leidenfrost effect is a physical phenomenon in which a liquid, in near contact with a mass significantly hotter than the liquid's boiling point, produces an insulating vapor layer keeping that liquid from boiling rapidly. Due to this ‘repulsive force,’ the droplet hovers over the surface rather than making physical contact with it. This is most commonly seen when cooking: one sprinkles drops of water in a pan to gauge its temperature: if the pan's temperature is at or above the Leidenfrost point, the water skitters across the pan and takes longer to evaporate than in a pan below the temperature of the Leidenfrost point (but still above boiling temperature). The effect is also responsible for the ability of liquid nitrogen to skitter across floors. It has also been used in some potentially dangerous demonstrations, such as dipping a wet finger in molten lead or blowing out a mouthful of liquid nitrogen, both enacted without injury to the demonstrator. The latter is potentially lethal, particularly should one accidentally swallow the liquid nitrogen.
Read more on Nature: Leidenfrost levitation: beyond droplets
No comments:
Post a Comment