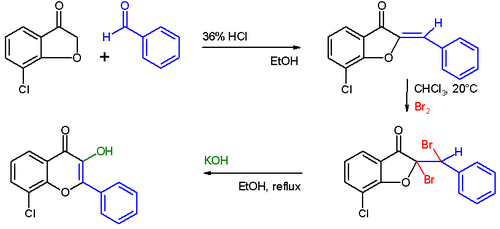
The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.
The first step in this procedure is an acid catalyzed aldol condensation between benzaldehyde and a 3-oxypentanone to an o-hydroxychalcone. Bromination of the alkene group gives a dibromo-adduct whichrearranges to the flavonol by reaction with potassium hydroxide.

Flavonols are a class of flavonoidsthat have the 3-hydroxyflavonebackbone (IUPAC name : 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions thephenolic -OH groups. They are distinct from flavanols (with "a") such as catechin, another class of flavonoids.
No comments:
Post a Comment