
Cyanocarbons are organic compounds bearing enough cyano functional groups to significantly alter their chemical properties.
Important cyanocarbons:
- Tetracyanoethylene, which readily reduces to a stable anion, unlike most ethene derivatives
- Pentacyanocyclopentadiene, which forms a stable anion by ionization of the C-H bond.
- Tetracyanoethylene oxide, an electrophilic epoxide that undergoes ready scission of its C-C bond.
- Tetracyanoquinodimethane, C6H4-1,4-(C(CN)2)2, a powerful electron acceptor.
- Cyanoform (tricyanomethane), (NC)3CH
Tetracyanoethylene (TCNE),
 more correctly ethenetetracarbonitrile, is a clear colored organic compoundconsisting of ethylene with the four hydrogen atom replaced with cyanogroups. It is an important member of the cyanocarbons.
more correctly ethenetetracarbonitrile, is a clear colored organic compoundconsisting of ethylene with the four hydrogen atom replaced with cyanogroups. It is an important member of the cyanocarbons.
TCNE is often used as an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems linked (conjugated) to the central C=C double bond, gives rise to an excellent acceptor. Thus, treatment of TCNE with iodide salts gives the radical anion:
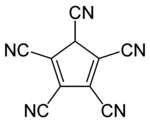 Pentacyanocyclopentadiene is aderivative of cyclopentadiene with five cyano groups with the molecular formula C5H(CN)5. The corresponding anion,pentacyanocyclopentadienide, is a ligand with the molecular formula C5(CN)5−. In contrast to other anions based on a C5 ring unit it binds to metals through the pendant cyano groups rather than the C5 ring. The anion was first synthesised by Webster in the 1960s and its conjugate acid much later on. More recently Wright has discovered its extensive coordination chemistry.
Pentacyanocyclopentadiene is aderivative of cyclopentadiene with five cyano groups with the molecular formula C5H(CN)5. The corresponding anion,pentacyanocyclopentadienide, is a ligand with the molecular formula C5(CN)5−. In contrast to other anions based on a C5 ring unit it binds to metals through the pendant cyano groups rather than the C5 ring. The anion was first synthesised by Webster in the 1960s and its conjugate acid much later on. More recently Wright has discovered its extensive coordination chemistry.

Like TCNE, TCNQ is easily reduced to give a blue-coloured radical anion. The reduction potential is about −0.3 V vs the ferrocene/ferrocenium couple. This property is exploited in the development of charge transfer salts. TCNQ also forms complexes with electron rich metal complexes.

 A Bechgaard salt is any one of a number of organic charge-transfer complexes that exhibit superconductivity at low temperatures.They are named for chemist Klaus Bechgaard, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist Denis Jérôme. Most Bechgaard salt superconductors are extremely low temperature, and lose superconductivity above the 1–2 K range, although the most successful compound in this class superconducts up to almost 12 K.
Tetrathiafulvalene (TTF) center typical in organic superconductors
A Bechgaard salt is any one of a number of organic charge-transfer complexes that exhibit superconductivity at low temperatures.They are named for chemist Klaus Bechgaard, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist Denis Jérôme. Most Bechgaard salt superconductors are extremely low temperature, and lose superconductivity above the 1–2 K range, although the most successful compound in this class superconducts up to almost 12 K.
Tetrathiafulvalene (TTF) center typical in organic superconductors
All Bechgaard salts have a variation on a single tetrathiafulvalene motif—different superconductors have been made with appendages to the motif, or using a tetraselenafulvalene center instead (which is a related compound), but all bear this general structural similarity.
 Tetrathiafulvalene is an organosulfur compound with the formula (H2C2S2C)2. Studies on this heterocyclic compound contributed to the development ofmolecular electronics. TTF is related to the hydrocarbonfulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.
Tetrathiafulvalene is an organosulfur compound with the formula (H2C2S2C)2. Studies on this heterocyclic compound contributed to the development ofmolecular electronics. TTF is related to the hydrocarbonfulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.
Klaus Bechgaard (born March 5, 1945 in Copenhagen,Denmark) is a Danish scientist and chemist, noted for being one of the first scientists in the world to synthesize a number of organic charge transfer complexes and demonstrate theirsuperconductivity, threreof the name Bechgaard salt. These salts all exhibit superconductivity at low temperatures.
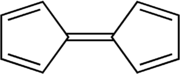 Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compoundsnaphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compoundsnaphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
- C2(CN)4 + I− → [C2(CN)4]− + 0.5 I2

Tetracyanoethylene oxide

Tetracyanoquinodimethane (TCNQ)
is the organic compound with the formula (NC)2CC6H4C(CN)2. Thiscyanocarbon is a relative of para-quinone is an electron acceptor that is used to prepare charge transfer salts, which are of interest in molecular electronics.
Like TCNE, TCNQ is easily reduced to give a blue-coloured radical anion. The reduction potential is about −0.3 V vs the ferrocene/ferrocenium couple. This property is exploited in the development of charge transfer salts. TCNQ also forms complexes with electron rich metal complexes.

Cyanoform (tricyanomethane)
is acyanocarbon and derivative of methane with three cyano groups. For many years, chemists have been unable to isolate this compound as a neat, free acid. However, in September 2015, reports surfaced of a successful isolation.
See also:

All Bechgaard salts have a variation on a single tetrathiafulvalene motif—different superconductors have been made with appendages to the motif, or using a tetraselenafulvalene center instead (which is a related compound), but all bear this general structural similarity.


Further reading:
Addition:
Quite interesting new star-shaped molecule: cyanostar a cyanostilbene-based macrocycle with anion-binding abilities.


No comments:
Post a Comment