Menthol is an organic compound made synthetically or obtained from corn mint, peppermint, or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration. Menthol has local anesthetic and counter irritant qualities, and it is widely used to relieve minort hroat irritation. Menthol also acts as a weak kappa opioid receptor agonist.
 The κ-opioid receptor (KOR) is aprotein that in humans is encoded by theOPRK1 gene. The KOR is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering pain perception,consciousness, motor control, and mood.
The κ-opioid receptor (KOR) is aprotein that in humans is encoded by theOPRK1 gene. The KOR is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering pain perception,consciousness, motor control, and mood.
 Myrcene, or β-myrcene, is an olefinicnatural organic hydrocarbon. It is more precisely classified as a monoterpene. Monoterpenes are dimers of isoprenoid precursors, and myrcene is one of the most important. It is a component of theessential oil of several plants including bay, cannabis, ylang-ylang, wild thyme, parsley, and hops. It is produced mainly semi-synthetically from myrcia, from which it gets its name.
Myrcene, or β-myrcene, is an olefinicnatural organic hydrocarbon. It is more precisely classified as a monoterpene. Monoterpenes are dimers of isoprenoid precursors, and myrcene is one of the most important. It is a component of theessential oil of several plants including bay, cannabis, ylang-ylang, wild thyme, parsley, and hops. It is produced mainly semi-synthetically from myrcia, from which it gets its name.
 Takasago International Corporation(高砂香料工業株式会社 Takasago Kōryō Kōgyō Kabushiki-gaisha) (TYO: 4914) is a major international producer of flavours and fragrances headquartered in Japan, with presence in 24 countries worldwide and net sales of $900 million in 2006.
Takasago International Corporation(高砂香料工業株式会社 Takasago Kōryō Kōgyō Kabushiki-gaisha) (TYO: 4914) is a major international producer of flavours and fragrances headquartered in Japan, with presence in 24 countries worldwide and net sales of $900 million in 2006.


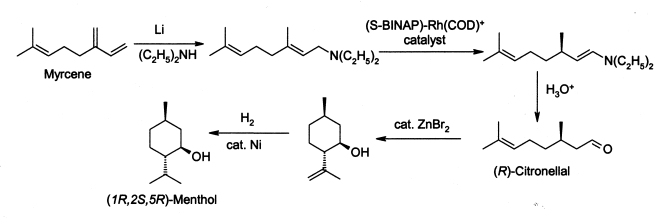
Menthol is manufactured as a single enantiomer (94% ee) on the scale of 3,000 tons per year by Takasago International Corporation. The process involves an asymmetric synthesis developed by a team led by Ryōji Noyori, who won the 2001 Nobel Prize for Chemistry in recognition of his work on this process:



- The process begins by forming an allylic amine from myrcene, which undergoes asymmetric isomerisation in the presence of a BINAP rhodium complex to give (after hydrolysis) enantiomerically pure R-citronellal. This is cyclised by a carbonyl-ene-reaction initiated by zinc bromide to isopulegol, which is then hydrogenated to give pure (1R,2S,5R)-menthol.
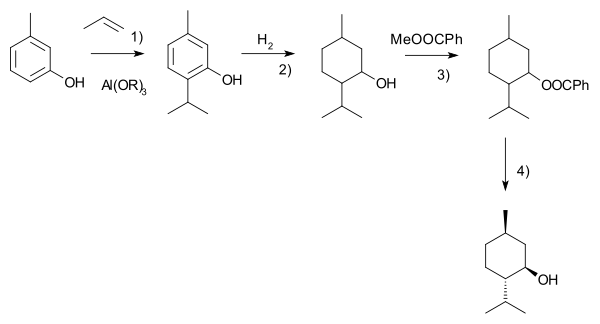
Another commercial process is the Haarmann-Reimer process. This process starts from m-cresol which is alkylated with propene to thymol. This compound is hydrogenated in the next step. Racemic menthol is isolated by fractional distillation. The enantiomers are separated by chiral resolution in reaction with methyl benzoate, selective crystallisation followed by hydrolysis.
No comments:
Post a Comment