 | |
Chemical identification, isolation, and synthesis
In 1819, the German chemist Friedlieb Ferdinand Rungeisolated relatively pure caffeine for the first time; he called it "Kaffebase" (i.e. a base that exists in coffee).According to Runge, he did this at the behest of Johann Wolfgang von Goethe. In 1821, caffeine was isolated both by the French chemist Pierre Jean Robiquet and by another pair of French chemists, Pierre-Joseph Pelletierand Joseph Bienaimé Caventou, according to Swedish chemist Jöns Jacob Berzelius in his yearly journal. Furthermore, Berzelius stated that the French chemists had made their discoveries independently of any knowledge of Runge's or each other's work. However, Berzelius later acknowledged Runge's priority in the extraction of caffeine, stating: "However, at this point, it should not remain unmentioned that Runge (in his Phytochemical Discoveries, 1820, pages 146–147) specified the same method and described caffeine under the name Caffeebase a year earlier than Robiquet, to whom the discovery of this substance is usually attributed, having made the first oral announcement about it at a meeting of the Pharmacy Society in Paris."
Pelletier's article on caffeine was the first to use the term in print (in the French form Caféine from the French word for coffee: café). It corroborates Berzelius's account:
Robiquet was one of the first to isolate and describe the properties of pure caffeine,whereas Pelletier was the first to perform an elemental analysis.
In 1827, M. Oudry isolated "théine" from tea, but it was later proved by Mulder and by Carl Jobst that theine was actually caffeine.
In 1895, German chemist Hermann Emil Fischer (1852–1919) first synthesized caffeine from its chemical components (i.e. a "total synthesis"), and two years later, he also derived the structural formula of the compound. This was part of the work for which Fischer was awarded the Nobel Prize in 1902.
Chemical properties
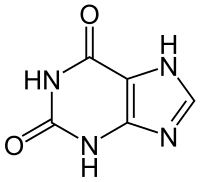
The xanthine core of caffeine contains two fused rings, a pyrimidinedione andimidazole. The pyrimidinedione in turn contains two amide functional groups that exist predominately in a zwitterionic resonance the location from which the nitrogen atoms are double bonded to their adjacent amide carbons atoms. Hence all six of the atoms within the pyrimidinedione ring system are sp2 hybridized and planar. Therefore, the fused 5,6 ring core of caffeine contains a total of ten pi electrons and hence according to Hückel's rule is aromatic.
Biosynthesis
Caffeine may be synthesized from dimethylurea and malonic acid, but is rarely obtained from synthesis since it is readily available as a byproduct of decaffeination.
Decaffeination
Extraction of caffeine from coffee, to produce caffeine and decaffeinated coffee, can be performed using a number of solvents.Benzene, chloroform, trichloroethylene, anddichloromethane have all been used over the years but for reasons of safety, environmental impact, cost, and flavor, they have been superseded by the following main methods:
- Water extraction: Coffee beans are soaked in water. The water, which contains many other compounds in addition to caffeine and contributes to the flavor of coffee, is then passed through activated charcoal, which removes the caffeine. The water can then be put back with the beans and evaporated dry, leaving decaffeinated coffee with its original flavor. Coffee manufacturers recover the caffeine and resell it for use in soft drinks and over-the-counter caffeine tablets.
- Supercritical carbon dioxide extraction: Supercritical carbon dioxide is an excellent nonpolar solvent for caffeine, and is safer than the organic solvents that are otherwise used. The extraction process is simple: CO2 is forced through the green coffee beans at temperatures above 31.1 °C and pressures above 73 atm. Under these conditions, CO2 is in a "supercritical" state: It has gaslike properties that allow it to penetrate deep into the beans but also liquid-like properties that dissolve 97–99% of the caffeine. The caffeine-laden CO2 is then sprayed with high pressure water to remove the caffeine. The caffeine can then be isolated bycharcoal adsorption (as above) or by distillation, recrystallization, or reverse osmosis.
- Extraction by organic solvents: Certain organic solvents such as ethyl acetatepresent much less health and environmental hazard than chlorinated and aromatic organic solvents used formerly. Another method is to use triglyceride oils obtained from spent coffee grounds.
"Decaffeinated" coffees do in fact contain caffeine in many cases — some commercially available decaffeinated coffee products contain considerable levels. One study found that decaffeinated coffee contained 10 mg of caffeine per cup, compared to approximately 85 mg of caffeine per cup for regular coffee.
Analogs
Some analog substances have been created which mimic caffeine's properties with either function or structure or both. Of the latter group are the xanthines DMPX and 8-chlorotheophylline, which is an ingredient in dramamine. Members of a class of nitrogen substituted xanthines are often proposed as potential alternatives to caffeine. Many other xanthine analogues constituting the adenosine receptor antagonist class have also been elucidated.
Some other caffeine analogs:


Further reading:




I was diagnosed as HEPATITIS B carrier in 2013 with fibrosis of the
ReplyDeleteliver already present. I started on antiviral medications which
reduced the viral load initially. After a couple of years the virus
became resistant. I started on HEPATITIS B Herbal treatment from
ULTIMATE LIFE CLINIC (www.ultimatelifeclinic.com) in March, 2020. Their
treatment totally reversed the virus. I did another blood test after
the 6 months long treatment and tested negative to the virus. Amazing
treatment! This treatment is a breakthrough for all HBV carriers.