There are several Akabori amino acid reactions, which are named after Shiro Akabori (1900–1992), a Japanese chemist.
In the first reaction, an α-amino acid is oxidised by heating it with an oxidizing sugar. This reaction has been used to synthesize dichlorophthalimido derivatives for the analysis of peptides, since the mass spectra of those derivatives are easily recognized.
In the second reaction, an α-amino acids and esters are reduced by sodium amalgam and ethanolic HCl to give α-amino aldehydes. This process is conceptually similar to the Bouveault–Blanc reduction except that is pauses at the aldehyde stage.

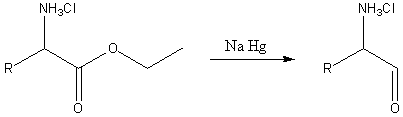
No comments:
Post a Comment